Supervisor: Dr Karen Syres
Contact : ksyres@uclan.ac.uk, Phone : +44 (0)1772 893850
Ionic liquids (ILs) consist of ions held together by a strong Coulomb potential and can remain liquid up to around 400 K. The combination of cations and anions can be tuned to create ILs with desired properties. ILs have very low vapour pressures and as a result can be studied in ultra-high vacuum conditions. This has opened up an exciting new avenue of research for liquid surface science. ILs are being investigated for applications such as gas capture and separation, electrolyte in batteries and photovoltaic devices, catalysis, lubrication and nanoparticle growth. There has been an explosion of research into ILs over the last few years but there is very little research into the fundamental interactions of ILs at surfaces, which is important to many of their applications.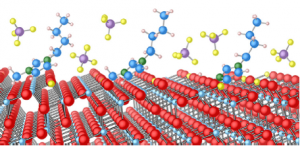
In this project you will investigate the structure and interactions of ILs at surfaces and interfaces. You will investigate how ILs interact with gas molecules with a view towards gas capture applications and you will study the interaction of ILs with oxide surfaces (such as ZnO and TiO2) with a view towards photovoltaic and catalysis applications.
You will use techniques such as X-ray photoelectron spectroscopy, X-ray absorption spectroscopy and diffraction techniques primarily at synchrotron facilities around Europe, to determine the structure, bonding and charge dynamics at the interfaces. For the study of gas capture in ILs you will carry out experiments at facilities which allow photoelectron spectroscopy measurements to be taken at near-ambient pressures (~10 mbar). Near-ambient pressure photoelectron experiments are a very recent development in surface science and for this project they will create a more realistic environment where the IL is in dynamic equilibrium with the surrounding gas. This fundamental understanding of IL surfaces and interfaces is vital to improving their efficiency in technological applications.
